This month's Accretionary Wedge (hosted by Magma Cum Laude) is about earth science outreach, so I'd like to write a bit about the practical courses our university (BTU – Brandenburg University of Technology at Cottbus) provides for secondary school students in 11th and 12th grade.
Our department (Environmental Geology) offers such practicals since 2007, but other departments have started earlier. Students of the secondary schools “Max-Steenbeck-Gymnasium” here in Cottbus and Carl-Friedrich-Gauß-Gymnasium1 in Frankfurt (Oder) attend practicals here as part of the “Forschungs-Bildungs-Kooperation” programme (FBK). Sorry, the schools' and FBK's websites are in German only.
Both schools have a special focus on mathematics, natural science and technology, and the students choose a practical course topic according to their elected “Leistungskurse” (intensive courses). In our case, these were either biology or chemistry. Sadly, geography intensive courses2 are not part of the FBK programme.
Up to now, we had three groups and topics:
- Physical properties of highly concentrated solutions of various salts (Gauß Gymnasium)
- Water quality of a small ditch flowing through a fen (Steenbeck Gymnasium)
- Release of nutrients from strongly degraded peat soils (Steenbeck Gymnasium)
Highly concentrated salt solutions
This was a project quite close to pure chemistry. The students prepared solutions of NaCl, MgCl2, CaCl2, MgSO4, and a few two-salt mixtures at various, high molalities (mostly in the range of 0.5 to 5 mol/kg) and investigated how density and electrical conductivity differ between the salts and how they change a different temperatures, concentrations and mixing ratios.
They found out that some relationsships are quite linear or at least monotonic, e.g. conductivity versus temperature or density versus concentration, but are not the same (different slope, different intersect) for different salts and concentrations. But they realised that there are also non-linear dependencies, e.g. conductivity versus concentration: at very high concentrations, conductivity decreases again (obstruction of ions).
Everytime, and always too late, I realise I didn't take enough photos of our work, so you have to be satisfied with one of the resulting diagrams. For the following topics, the situation is a bit better luckily.
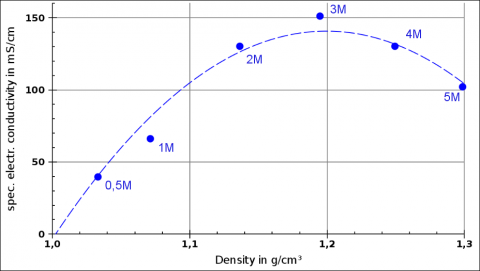
Example from Gauß Gymansium students' experiments: Density and electrical conductivity for MgCl2 solutions of different molalities. “1M” = 1 mol/kg.
Quality of water flowing through a fen
My first group of students from Steenbeck Gymnasium measured discharge of a small ditch flowing through a (slightly degraded) valley fen at its beginning and end. Also, measured pH, conductivity, oxygen, temperature and alkalinity on-site, and collected water samples and analysed them for nitrogen (total, nitrate, ammonia), phosphate, dissolved organic carbon, chloride, boron (as sewage indicator).
Sadly, I couldn't find a decent photo of the discharge measurements.
Again, I made some pictures of the sample preparation, but not of the analysis proper.
With these data, they could balance the fluxes entering and leaving the system. For example, denitrification within the system can be seen nicely.
Nutrient release from strongly degraded peat
This year's students took soil samples from another fen site, where the peat ist strongly degraded after long time of drainage and agricultural use. Degradation not only means a loss of soil, but also mineralisation of organic matter, i.e. nutrients like nitrogen and phosphorus which had formerly been bound in large organic molecules are now present in mobile forms like nitrate. These could then be leached out, especially when the water table rises again, and affect downstream surface water (eutrophication).

Borehole in strongly degraded peat. (Brighter material at the tip of the auger is the underlying finesand.)
In a simple laboratory setup, we try to find out what could happen after rewetting. Soil samples are extracted with water under controlled conditions (fixed temperature, fixed soil/water ratio, bottles gently rotated for 24 hours).
After that, the students measure physico-chemical parameters (pH, el. conductivity, redox) and analyse the eluate for nutrient concentrations (nitrogen and phosphorus species).
We are still working on the project, so there are no nice results to present right now.
- Gymnasium is the school that is intended as a preparation for university. Have a look at the Wikipedia article on Germany's “slightly complicated” school system. [↩]
- And there's no such thing as a geology intensive course. [↩]







Post a Comment